Introduction: Ciltacabtagene autoleucel (cilta-cel) is a chimeric antigen receptor-T cell (CAR-T) immunotherapy approved for patients with relapsed or refractory multiple myeloma (RRMM). In the phase 3 trial, CARTITUDE-4 (NCT04181827), cilta-cel demonstrated better efficacy than physician's choice (daratumumab plus pomalidomide and dexamethasone [DPd] or pomalidomide plus bortezomib and dexamethasone [PVd]) with an overall response rate (ORR) of 84.6% versus 67.3% and ≥complete response (CR) of 73.1% versus 21.8% (San-Miguel et al. New Engl J Med. 2023). The present analysis assessed the value of cilta-cel and physician's choice treatments within the CARTITUDE-4 trial from a US combined commercial and Medicare payer perspective using a novel cost per responder (CPR) model that incorporates efficacy and total treatment costs.
Methods: A CPR analysis was conducted from a mixed payer perspective (67.7% commercial, 23.3% Medicare), comparing the direct medical cost per patient receiving cilta-cel versus physician's choice (87% DPd and 13% PVd, per distribution in the trial) over 25.4 months, the maximum observed period for the physician's choice arm in the CARTITUDE-4 trial. The model was developed using progression-free survival (PFS), overall survival (OS), time to next treatment, CR, stringent CR (sCR), and ORR endpoints from the CARTITUDE-4 trial to inform health status. A standard parametric approach was taken to extrapolate the PFS and OS beyond the trial period for each arm for analysis scenarios with longer follow-up. Resource use and costs associated with the administration process for each treatment were included: apheresis, bridging therapy, pre-conditioning treatment, infusion, drug acquisition, other medications (infection prophylaxis, antihistamine, and antipyretic), and monitoring (eg, labs, vital sign assessment, hematologist visit) for CAR-T; drug acquisition, administration, other medications, and monitoring for physician's choice. The base case assumed CAR-T infusion was entirely administered in the inpatient setting, and an additional scenario was included that considered the outpatient infusion setting (70% inpatient and 30% outpatient). Costs of managing grade 3 to 4 AEs and grade 1 to 4 AEs for cytokine release syndrome (CRS) and neurotoxicity were included. Costs of subsequent therapies were assigned following disease progression, and costs of terminal care were also considered upon death events. Model outcomes included total cost per treated patient, total cost per complete responder, and cost per month in PFS compared between cilta-cel and physician's choice of therapy. All costs were adjusted to 2023 US dollars based on the consumer price index for medical care.
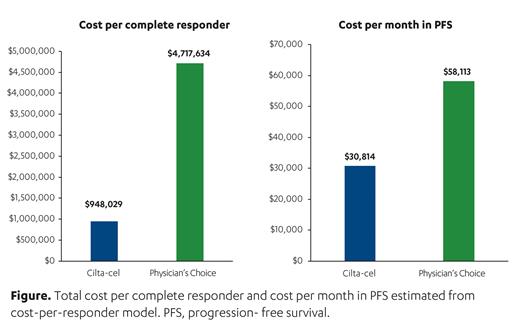
Results: The total cost (PFS and post-progression survival [PPS] combined) per treated patient was estimated as $693,009 for cilta-cel and $1,028,444 for physician's choice over 25.4 months. Total estimated costs per treated patient were lower for cilta-cel than physician's choice during PFS and PPS separately ($581,108 vs $765,169 and $111,901 vs $263,275, respectively). Treatment acquisition and subsequent treatment costs are key drivers of the total costs during PFS and PPS, respectively ($451,318 and $103,101 for cilta-cel; $726,356 and $249,005 for physician's choice). Total cost per complete responder was estimated to be lower for cilta-cel compared with physician's choice ($948,029 vs $4,717,634) (Figure). Total estimated cost per month in PFS was $30,814 for cilta-cel versus $58,113 for physician's choice (Figure). Scenario analysis with a lower percentage of patients receiving CAR-T infusion in inpatient settings led to lower cost per complete responder and lower cost per month in PFS for cilta-cel (70% inpatient and 30% outpatient, $940,431 and $30,520, respectively).
Conclusions: Overall, cilta-cel offers substantial clinical and economic benefit for patients with RRMM. The CPR analysis of data from CARTITUDE-4 estimated that, for patients with RRMM, cost per complete responder, and cost per month in PFS for cilta-cel were remarkably lower compared to physician's choice (DPd or PVd). Treatment acquisition and subsequent treatment costs appear to be key drivers for total direct medical costs.
Disclosures
Hansen:BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; Survivorship: Honoraria; Onc Live: Honoraria; International Myeloma Society Young Investigator Award: Research Funding; Pfizer: Consultancy; Pentecost Family Myeloma Research Center: Research Funding; BMS: Consultancy, Research Funding; Janssen: Consultancy; Karyopharm: Consultancy, Research Funding; BMS MM ASH Steering Committee: Membership on an entity's Board of Directors or advisory committees; MM Pfizer Advisory Board: Membership on an entity's Board of Directors or advisory committees. Lu:Janssen Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Castaneda:Moffitt Cancer Center: Current Employment; Adaptive Biotechnologies: Speakers Bureau. Sorensen:Evidera: Current Employment. Usmani:SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Moderna: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; TeneoBio: Membership on an entity's Board of Directors or advisory committees; Array Biopharma: Research Funding; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; EdoPharma: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Huo:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company. Jagannath:Mount Sinai Hospital: Current Employment; Bristol Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Caribou Biosciences: Consultancy; Takeda: Consultancy; Regeneron: Consultancy; DMC: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy; IMS: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; ASH: Membership on an entity's Board of Directors or advisory committees; SOHO: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal